 Last month, FDA issued a Request for Information (RFI) in the Federal Register seeking information and comments from interested stakeholders regarding “critical scientific challenges and opportunities to advance the development of individualized cellular and gene therapies (CGTs).” Individualized CGTs are therapies “developed for a single patient (or a very small number of patients) based on designing or engineering a product that specifically targets the mechanism underlying a patient’s (or small number of patients’) illness.”
Last month, FDA issued a Request for Information (RFI) in the Federal Register seeking information and comments from interested stakeholders regarding “critical scientific challenges and opportunities to advance the development of individualized cellular and gene therapies (CGTs).” Individualized CGTs are therapies “developed for a single patient (or a very small number of patients) based on designing or engineering a product that specifically targets the mechanism underlying a patient’s (or small number of patients’) illness.”
FDA’s request focuses on three core areas:
1. Manufacturing: Manufacturing and product quality challenges and opportunities for individualized CGTs in light of, for example, small batch sizes, tailoring of batches to individual patients, and need for rapid testing and release.
On this topic, FDA asks:
- Given the challenges to develop consistent manufacturing strategies for CGTs designed for a very small number of patients or an individual patient, how can manufacturers leverage their prior experience manufacturing one CGT to support subsequent development and approval of another related, but distinct CGT (potential areas for leveraging may include manufacturing process validation, control strategy, assay validation, and drug product stability studies)?
- When the batch size of a CGT is very small, what are some challenges and solutions regarding the volume of product (or number of vials) needed for batch release testing, stability testing, retention of reserve samples, and comparability studies?
- What are some challenges and solutions for individualized CGTs that need to be tested and released rapidly, either because the product has a very short shelf life or because the patient’s clinical status may be rapidly declining and treatment is urgently needed?
- For many individualized CGT products, each batch is tailored to an individual patient (e.g., autologous CAR–T cells, tumor neoantigen vaccines, certain genome editing products). For such products, what are some challenges and solutions for assuring that each batch has adequate potency to achieve the intended therapeutic effect?
- What are some challenges and solutions for individualized genome editing products that aim to treat monogenic diseases for which the target gene has different mutations in different patients?
2. Nonclinical development: The use of nonclinical data to support individualized CGTs, considering the lack of relevant animal models in many instances, the uniqueness or limited applicability of individualized CGTs, and the potential of using prior knowledge from other CGTs—for example, where gene therapy vector products use the same vector backbone.
On this topic, FDA asks:
- What nonclinical studies could be leveraged in support of a related product using similar technologies? What nonclinical studies are important to conduct with each final clinical product?
- What nonclinical development approaches could be considered when there are no relevant animal models or animal models are unable to replicate each individual disease/condition?
- For patient-specific products where evaluating each individual product is infeasible or impractical, what is the role for nonclinical studies conducted with representative product(s)?
- What are the opportunities and challenges with using computational approaches to support nonclinical development?
3. Clinical Development: Clinical development of individualized CGTs, considering for example the infeasibility (for ethical or other reasons) of conducting randomized controlled studies, novel endpoints, and limitations in statistical analyses.
On this topic, FDA asks:
- What are challenges and strategies/opportunities with interpreting efficacy data from individual patients (including expanded access) and small groups of patients? What opportunities are there in leveraging prior and/or collective experiences?
- What strategies can be utilized to accumulate and interpret safety data in personalized/individualized CGTs?
- For genetic disorders with clear genotype-phenotype associations for disease manifestations or severity, what opportunities are there for tailoring treatments and study design to specific genotypes/phenotypes?
FDA also requested input on several additional significant questions:
- What additional major scientific challenges to advance the development of individualized CGTs should be considered?
- What existing best practices or scientific approaches should be leveraged to address any of these challenges? Are there specific opportunities for collaborations to advance the development of individualized CGTs?
- Are there specific areas where flexibility in regulatory approaches would improve the feasibility of developing and commercializing individualized CGTs?
Comments are due on November 20, 2023.
At the end of last month, FDA also announced a pilot program “to help further accelerate development of rare disease therapies.” The program, titled Support for clinical Trials Advancing Rare disease Therapeutics (“START”), will include selected sponsors with an active IND for products meeting certain eligibility requirements. Products regulated by CBER are eligible for the program only if they are a gene or cell therapy treatment for a rare disease or condition that is “likely to lead to significant disability or death within the first decade of life.” Products regulated by CDER are eligible only if they are “intended to treat rare neurodegenerative conditions, including those of rare genetic metabolic type.” Participants selected for the pilot program will “be able to obtain frequent advice and regular ad-hoc communication with FDA staff to address product-specific development issues, including, but not limited to, clinical study design, choice of control group and fine-tuning the choice of patient population.”
FDA will accept applications to the START program beginning January 2, 2024 and until March 1, 2024.
 On April 18, U.S. Food and Drug Administration (FDA) Commissioner Marty Makary announced plans to roll-out a new approval pathway for rare disease drugs. Commissioner Makary’s comments build on sentiments expressed across both the patient community and industry that rare disease drug development needs greater regulatory flexibility in order to speed access to treatments for patients with no or limited options. This is an initiative that has also been trumpeted by Janet Woodcock, former Principal Deputy Commissioner and Acting Commissioner of the FDA, in her work since retiring from the FDA. Prior legislative proposals (including the “Promising Pathway Act” proposed in 2024) have attempted to create a time-limited conditional approval pathway in the rare disease space, and Commissioner Makary’s remarks may signal a renewed push for action.
On April 18, U.S. Food and Drug Administration (FDA) Commissioner Marty Makary announced plans to roll-out a new approval pathway for rare disease drugs. Commissioner Makary’s comments build on sentiments expressed across both the patient community and industry that rare disease drug development needs greater regulatory flexibility in order to speed access to treatments for patients with no or limited options. This is an initiative that has also been trumpeted by Janet Woodcock, former Principal Deputy Commissioner and Acting Commissioner of the FDA, in her work since retiring from the FDA. Prior legislative proposals (including the “Promising Pathway Act” proposed in 2024) have attempted to create a time-limited conditional approval pathway in the rare disease space, and Commissioner Makary’s remarks may signal a renewed push for action. On October 4, 2024, a US House version of the revised Promising Pathway Act (PPA) 2.0 was introduced, sponsored by Rep. Bruce Westerman (R-AR). The bill (
On October 4, 2024, a US House version of the revised Promising Pathway Act (PPA) 2.0 was introduced, sponsored by Rep. Bruce Westerman (R-AR). The bill ( Support Goodwin’s DC Life Sciences Team as they raise money for the National Organization for Rare Disorders (NORD) as part of its “Running for Rare” team at the annual 10K race held during the upcoming DC Marine Corps Marathon on October 27th.
Support Goodwin’s DC Life Sciences Team as they raise money for the National Organization for Rare Disorders (NORD) as part of its “Running for Rare” team at the annual 10K race held during the upcoming DC Marine Corps Marathon on October 27th. Last month, FDA issued a
Last month, FDA issued a 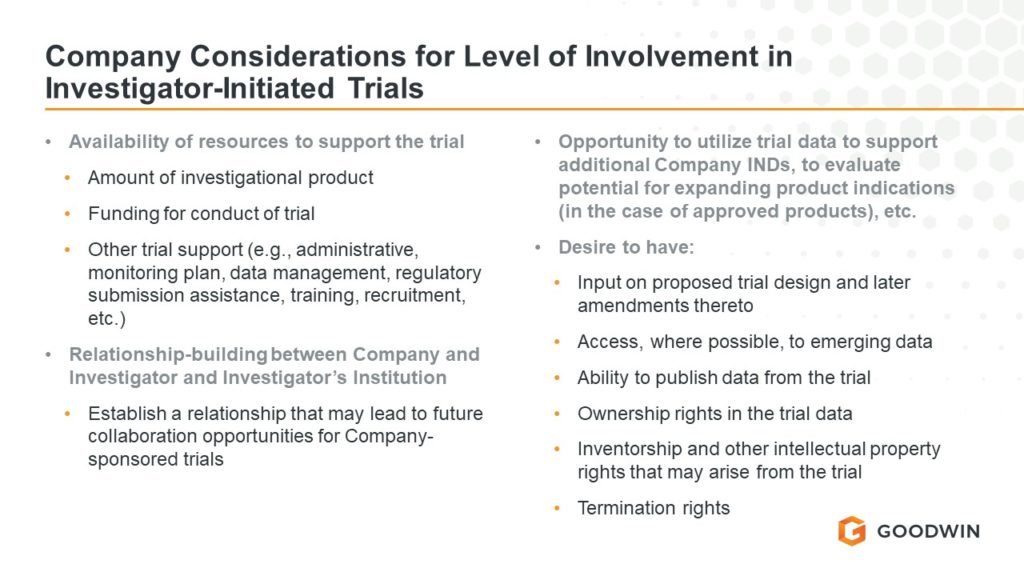
 In global observance of Rare Disease Day, Goodwin invites you to join us for a special awareness event on March 1, 2023 in our Boston office or virtually for those attending remotely to spotlight the critical work being done to address over 7,000 rare diseases that impact more than 300 million people globally.
In global observance of Rare Disease Day, Goodwin invites you to join us for a special awareness event on March 1, 2023 in our Boston office or virtually for those attending remotely to spotlight the critical work being done to address over 7,000 rare diseases that impact more than 300 million people globally.