NIH Again Refuses to Exercise March-In Rights to Control Drug Price
 In a letter dated March 21, 2023, the National Institutes of Health (“NIH”) again refused the request of petitioners to exercise march-in rights under the Bayh-Dole Act to control the price of a drug. Here, as before, the NIH found that the statutory criteria for the use of march-in rights were not satisfied by the petitioners.
In a letter dated March 21, 2023, the National Institutes of Health (“NIH”) again refused the request of petitioners to exercise march-in rights under the Bayh-Dole Act to control the price of a drug. Here, as before, the NIH found that the statutory criteria for the use of march-in rights were not satisfied by the petitioners.
March-in rights can permit the government to require a patent owner to grant additional licenses to the invention to avoid situations such as a company licensing the technology but then not commercializing it. The Bayh-Dole Act enumerates the circumstances under which march-in rights and the grant of additional licenses are warranted, for example, to achieve practical application of the invention or to alleviate health and safety needs that are not being reasonably satisfied.
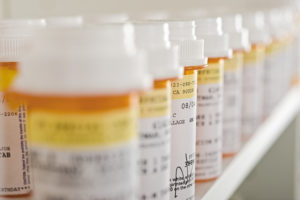
In November 2021, the Secretary of the Department of Health and Human Services (“HHS”) received a petition from individuals Robert Sachs and Clare Love requesting the exercise of march-in rights under the Bayh-Dole Act to lower the price of the prostate cancer drug, Xtandi (enzalutamide). The patented drug product was invented at the University of California, Los Angeles, with funding from the NIH and U.S. Army. Xtandi, which is marketed in the United States by Astellas and Pfizer, costs more in the U.S. than it does elsewhere including other high-income countries. Petitioners argued that drug price can forbid access, specifically at prices that are allegedly unreasonable, contrary to the Bayh-Dole Act.
While the NIH’s response letter expressed its concern about the high cost of drugs and the burden it places on patients, the letter explained the purpose of the Bayh-Dole Act is to promote the commercialization and public availability of government funded inventions. The overarching proposition of the Act is to permit recipients of federal government funding to retain ownership of patent rights and thereby commercialize the inventions by partnering with the private sector. Prior to the Bayh-Dole Act, most government funded inventions were not licensed or commercialized, including not one drug product.
The letter indicated that the NIH’s analysis found that Xtandi is widely available to the public. The NIH stated that consistent with past march-in determinations in response to petitions for controlling drug prices, practical application of the invention is evidenced by practice of the invention and the invention’s availability to the public. Astellas, the maker of Xtandi, estimated that more than 200,000 patients since 2012 were treated with the drug. Accordingly, the NIH concluded that the patent owner, the University of California, which licenses the patents to Astellas, meets the requirement for bringing Xtandi to practical application.
In addition, the NIH also stated that given the remaining patent life of the drug and the lengthy administrative procedure for the exercise of march-in rights, the NIH does not believe that the use of march-in rights would be an effective way at lowering the cost of the drug. Therefore, for these reasons, the NIH determined that march-in rights were not warranted in this situation.
The letter ends stating that the NIH and HHS would pursue a “whole of government approach,” informed by public input, to ensure the use of march-in rights is consistent with the Bayh-Dole Act, promotes commercialization of federally funded research, maximizes the potential for federally funded technologies to become products, and is in the interests of the American public. To that end, on the same day as the NIH letter, HHS and the Department of Commerce (“DOC”) announced a plan to review march-in authority as found in the Bayh-Dole Act with these same goals.
The NIH decision is in line with the several other petitions that have been filed for other drugs over the last few decades as well as previous petitions involving Xtandi. The exercise of march-in rights by a federal agency likely would have a negative impact on companies developing products invented using federal funding if investors believe that the price of such products could be controlled by the federal government based on public input. We will continue to monitor developments in this area, including for any recommendations from the HHS and DOC inter-agency working group on this important topic.
 Director Katherine Vidal of the U.S. Patent and Trademark Office (“USPTO”) issued a precedential review decision with respect to the interpretation of multiple dependent claims, in a case of first impression before the Patent and Trial Appeal Board (“PTAB”). In the review of the PTAB’s final written Decision and Order, the Director modified it consistent with her determination of the treatment of multiple dependent claims, which are claims that refer to and incorporate by reference more than one other claim.
Director Katherine Vidal of the U.S. Patent and Trademark Office (“USPTO”) issued a precedential review decision with respect to the interpretation of multiple dependent claims, in a case of first impression before the Patent and Trial Appeal Board (“PTAB”). In the review of the PTAB’s final written Decision and Order, the Director modified it consistent with her determination of the treatment of multiple dependent claims, which are claims that refer to and incorporate by reference more than one other claim. The U.S. Patent and Trademark Office (“USPTO”) published a Notice in the Federal Register announcing a new pilot program entitled, “Cancer Moonshot Expedited Examination Pilot Program” (the “Cancer Moonshot Program”) (87 Fed. Reg. 75608 (December 9, 2022)) (the “Notice”) to attempt to further accelerate innovation in the health and medical fields. Beginning on February 1, 2023, this new program will replace the Cancer Immunotherapy Pilot Program and expedite examination for a broader scope of technologies to prevent cancer and advance smoking cessation. The Cancer Moonshot Program is to support President Biden’s recently renewed Cancer Moonshot initiative, which set a new goal of reducing cancer death rate by at least 50% over the next 25 years.
The U.S. Patent and Trademark Office (“USPTO”) published a Notice in the Federal Register announcing a new pilot program entitled, “Cancer Moonshot Expedited Examination Pilot Program” (the “Cancer Moonshot Program”) (87 Fed. Reg. 75608 (December 9, 2022)) (the “Notice”) to attempt to further accelerate innovation in the health and medical fields. Beginning on February 1, 2023, this new program will replace the Cancer Immunotherapy Pilot Program and expedite examination for a broader scope of technologies to prevent cancer and advance smoking cessation. The Cancer Moonshot Program is to support President Biden’s recently renewed Cancer Moonshot initiative, which set a new goal of reducing cancer death rate by at least 50% over the next 25 years. After a recent reminder from the U.S. Patent and Trademark Office (USPTO) regarding the duties of disclosure and reasonable inquiry during examination of a patent application and a Request for Comments (RFC) on the USPTO initiatives to ensure “robustness and reliability” of patent rights,[1] the Director of the U.S. Patent and Trademark Office published a third notice in less than four months. The latest notice is in conjunction with the Food and Drug Administration (FDA) to further the discussion surrounding the patent practices of the pharmaceutical industry (
After a recent reminder from the U.S. Patent and Trademark Office (USPTO) regarding the duties of disclosure and reasonable inquiry during examination of a patent application and a Request for Comments (RFC) on the USPTO initiatives to ensure “robustness and reliability” of patent rights,[1] the Director of the U.S. Patent and Trademark Office published a third notice in less than four months. The latest notice is in conjunction with the Food and Drug Administration (FDA) to further the discussion surrounding the patent practices of the pharmaceutical industry ( The new Director of the U.S. Patent and Trademark Office (USPTO), Katherine Vidal, published a stern reminder regarding the duties of disclosure and reasonable inquiry during examination of a patent application, including reexamination, reissue, and proceedings before the Patent Trial and Appeal Board (PTAB) (87 FR 45764 (July 29, 2022)). The justification was to provide examiners and judges with all the material information needed to decide on patentability of a claimed invention. According to the USPTO, more robust and reliable patents should result, which is better for the public.
The new Director of the U.S. Patent and Trademark Office (USPTO), Katherine Vidal, published a stern reminder regarding the duties of disclosure and reasonable inquiry during examination of a patent application, including reexamination, reissue, and proceedings before the Patent Trial and Appeal Board (PTAB) (87 FR 45764 (July 29, 2022)). The justification was to provide examiners and judges with all the material information needed to decide on patentability of a claimed invention. According to the USPTO, more robust and reliable patents should result, which is better for the public.