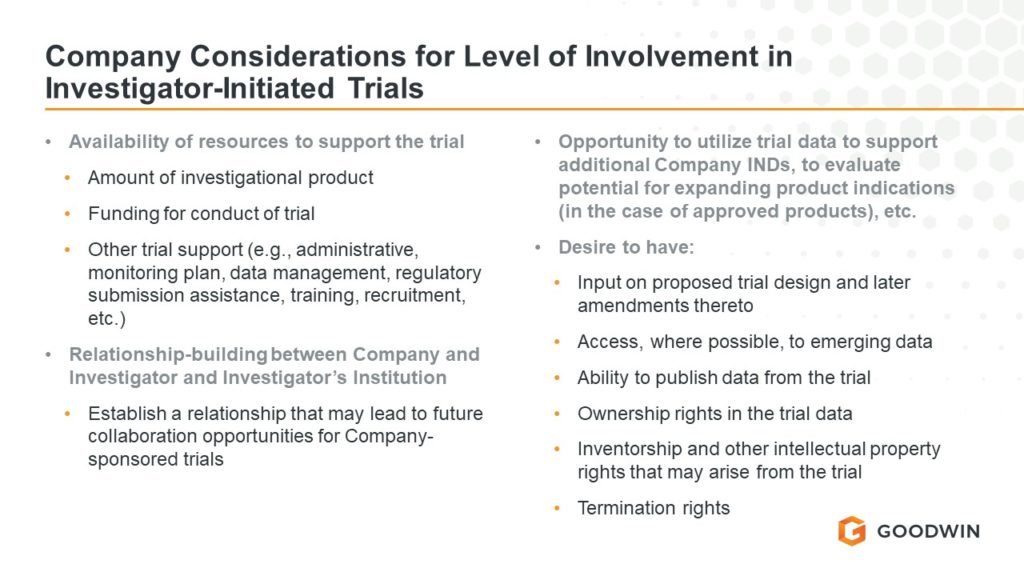
 When it comes to discussing medical devices regulated by the U.S. Food and Drug Administration (FDA), words such as “approved” and “cleared” cannot be used interchangeably as these terms carry a particular meaning. Similarly, creating an impression of approval of a device establishment or its devices because the establishment is registered with FDA also is prohibited. Long-standing regulatory provisions, 21 C.F.R. § 807.97 and 21 C.F.R. § 807.39, set forth, respectively, the FDA’s position that approval and clearance are not interchangeable and that device establishment registration does not denote approval of the establishment or its devices. Importantly, these provisions also highlight the consequences to industry for misusing terms when discussing the regulatory status of a device or a device establishment.
When it comes to discussing medical devices regulated by the U.S. Food and Drug Administration (FDA), words such as “approved” and “cleared” cannot be used interchangeably as these terms carry a particular meaning. Similarly, creating an impression of approval of a device establishment or its devices because the establishment is registered with FDA also is prohibited. Long-standing regulatory provisions, 21 C.F.R. § 807.97 and 21 C.F.R. § 807.39, set forth, respectively, the FDA’s position that approval and clearance are not interchangeable and that device establishment registration does not denote approval of the establishment or its devices. Importantly, these provisions also highlight the consequences to industry for misusing terms when discussing the regulatory status of a device or a device establishment.
When seeking to market a new device for which a premarket notification must be submitted to the FDA demonstrating that the device to be marketed is substantially equivalent to a legally marketed device, the submitter must obtain an order of substantial equivalence from the FDA, which is commonly referred to as a 510(k) clearance. Conversely, to market a new device for which a premarket approval application must be submitted to the FDA, the applicant must obtain FDA’s approval of the application. While FDA review and FDA action occur for both types of medical devices, the outcomes of clearance and approval are distinctly different and carry legal consequences. Specifically, 21 C.F.R. § 807.97 states that “[a]ny representation that creates an impression of official approval of a device because of complying with the premarket notification regulations is misleading and constitutes misbranding.” Additionally, 21 C.F.R. § 807.39 states that “[a]ny representation that creates an impression of official approval because of registration or possession of a registration number is misleading and constitutes misbranding.”
We researched Warning Letters in FDA’s Warning Letter Database and found that FDA issued four Warning Letters citing violations of § 807.97 since 2017 and thirteen Warning Letters citing violations of § 807.39 since 2017.
Many of the representations that FDA found to be misleading under § 807.97 were straightforward violations, such as language on product websites stating that cleared devices are “FDA approved,” or listings of device clearances under the heading “FDA Approvals.” In one instance, FDA found the website to be misleading under both § 807.39 and § 807.97 because the company claimed the device had been cleared by the FDA, when in fact it was marketing a 510(k) exempt device for an indication that would require a de novo authorization which the company had not obtained, and the website claimed the company maintained an active listing, which was hyperlinked to the company’s FDA Establishment Registration and Device Listing for only the 510(k) exempt device.
In response to the COVID-19 public health emergency, FDA issued twelve Warning Letters related to representations regarding masks and antibody tests that were found to be misleading under § 807.39. In virtually all of these instances, company websites displayed unofficial “certificates of FDA registration” issued by third parties which claimed to certify that the manufacturer had completed FDA Establishment Registration and Device Listing. These certificates often incorporated unauthorized reproductions of FDA’s logo and motifs of the U.S. flag, giving the impression of official government documents. FDA consistently found the display of these certificates to be misleading, even when they included ostensible “disclaimer” language stating that the certificates did not denote FDA endorsement or approval. FDA repeatedly found that these disclaimers did not adequately limit or otherwise mitigate the misleading impression of the certificates because they were phrased, designed, and placed in a manner where they could be easily overlooked.
These Warning Letters present a cautionary tale to all sponsors intending to market new medical devices. While sponsors may be tempted to claim their devices are approved by the FDA following the agency’s review of a premarket notification or upon completion of FDA Establishment Registration and Device Listing, § 807.97 and § 807.39 make clear that such claims will constitute misbranding. Sponsors can avoid § 807.97- and § 807.39-related Warning Letters and associated liability by carefully reviewing all of the language on their marketing materials and websites to ensure that none of their representations create the impression of official approval based on reference to a premarket notification submission or establishment registration.
Visit the Goodwin on Medtech hub to stay informed on important developments affecting medtech innovators and investors.
 For a healthcare or life sciences company settling a government enforcement action, the prospect of being subject to an independent monitor, independent review organization (IRO), or other government-mandated compliance expert may become a reality. (We collectively refer to all of these individuals and entities as monitors throughout this update.) Hiring an independent monitor is a sensitive topic, as a company subject to a monitorship is required to open up its records and files, financial information, proprietary and confidential materials, IT assets, and employees to a third party — often at frequent and regular intervals, and often for a period of five years — not to mention the potential multimillion-dollar expense associated with the engagement.
For a healthcare or life sciences company settling a government enforcement action, the prospect of being subject to an independent monitor, independent review organization (IRO), or other government-mandated compliance expert may become a reality. (We collectively refer to all of these individuals and entities as monitors throughout this update.) Hiring an independent monitor is a sensitive topic, as a company subject to a monitorship is required to open up its records and files, financial information, proprietary and confidential materials, IT assets, and employees to a third party — often at frequent and regular intervals, and often for a period of five years — not to mention the potential multimillion-dollar expense associated with the engagement.
 When it comes to discussing medical devices regulated by the U.S. Food and Drug Administration (FDA), words such as “approved” and “cleared” cannot be used interchangeably as these terms carry a particular meaning. Similarly, creating an impression of approval of a device establishment or its devices because the establishment is registered with FDA also is prohibited. Long-standing regulatory provisions,
When it comes to discussing medical devices regulated by the U.S. Food and Drug Administration (FDA), words such as “approved” and “cleared” cannot be used interchangeably as these terms carry a particular meaning. Similarly, creating an impression of approval of a device establishment or its devices because the establishment is registered with FDA also is prohibited. Long-standing regulatory provisions,  The Bioresearch Monitoring Program (BIMO), run by the U.S. Food and Drug Administration (FDA), oversees the conduct of on-site inspections and data audits of FDA-regulated research in support of new product development and marketing approvals. As a follow up to our
The Bioresearch Monitoring Program (BIMO), run by the U.S. Food and Drug Administration (FDA), oversees the conduct of on-site inspections and data audits of FDA-regulated research in support of new product development and marketing approvals. As a follow up to our  For emerging companies establishing their first supply chains, ensuring notification requirements in supply agreements for when commercial-stage manufacturing issues arise may not be top of mind. However, it is important for drug developers whose contracts enable continuation of a supply arrangement into the commercial-stage to be familiar with the U.S. Food and Drug Administration’s (FDA’s) field alert reporting (FAR) requirements for new drug application (NDA) and abbreviated new drug application (ANDA) holders to ensure adequate communication between developers and their suppliers.
For emerging companies establishing their first supply chains, ensuring notification requirements in supply agreements for when commercial-stage manufacturing issues arise may not be top of mind. However, it is important for drug developers whose contracts enable continuation of a supply arrangement into the commercial-stage to be familiar with the U.S. Food and Drug Administration’s (FDA’s) field alert reporting (FAR) requirements for new drug application (NDA) and abbreviated new drug application (ANDA) holders to ensure adequate communication between developers and their suppliers. The U.S. Food and Drug Administration’s (FDA’s) Office of Bioresearch Monitoring Operations (OBIMO) oversees domestic and foreign agency field inspections for clinical and non-clinical research. In particular, OBIMO manages the Bioresearch Monitoring (BIMO) Program which conducts onsite field inspections and data monitoring to ensure institution and industry compliance with FDA’s regulations relating to Good Clinical Practices (GCPs). These inspections can occur as a result of a marketing application submission, for general surveillance during an ongoing clinical trial, or as a result of a “for cause” reason. After an inspection, FDA investigators may issue a Form 483 to communicate any onsite findings of noncompliance with FDA’s regulations. BIMO also has authority to issue Warning Letters when the noncompliance FDA identifies is serious.
The U.S. Food and Drug Administration’s (FDA’s) Office of Bioresearch Monitoring Operations (OBIMO) oversees domestic and foreign agency field inspections for clinical and non-clinical research. In particular, OBIMO manages the Bioresearch Monitoring (BIMO) Program which conducts onsite field inspections and data monitoring to ensure institution and industry compliance with FDA’s regulations relating to Good Clinical Practices (GCPs). These inspections can occur as a result of a marketing application submission, for general surveillance during an ongoing clinical trial, or as a result of a “for cause” reason. After an inspection, FDA investigators may issue a Form 483 to communicate any onsite findings of noncompliance with FDA’s regulations. BIMO also has authority to issue Warning Letters when the noncompliance FDA identifies is serious. The pandemic has spared no industry. The life sciences industry knows this well and perhaps learned this lesson the hardest way during the pandemic when overseas supply shipments were delayed or, worse, when overseas manufacturing facilities were shut down because of government-mandated quarantines. Producing novel biologics is, unfortunately, not so easy to pick up and relocate, especially during a pandemic and even moreso when there are not enough domestic producers to begin with. As the life sciences industry continues to rapidly grow and mature in the U.S., life sciences clusters are growing and expanding into the next phase: biomanufacturing onsite and building their own self-sustaining supply operations. Learn more about the expansion of the domestic supply chain here.
The pandemic has spared no industry. The life sciences industry knows this well and perhaps learned this lesson the hardest way during the pandemic when overseas supply shipments were delayed or, worse, when overseas manufacturing facilities were shut down because of government-mandated quarantines. Producing novel biologics is, unfortunately, not so easy to pick up and relocate, especially during a pandemic and even moreso when there are not enough domestic producers to begin with. As the life sciences industry continues to rapidly grow and mature in the U.S., life sciences clusters are growing and expanding into the next phase: biomanufacturing onsite and building their own self-sustaining supply operations. Learn more about the expansion of the domestic supply chain here. On May 19, 2021, the U.S. Food and Drug Administration (FDA) released an
On May 19, 2021, the U.S. Food and Drug Administration (FDA) released an